Luminex and Unique Device Identification (UDI Regulations)
Luminex supports the FDA’s Unique Device Identification (UDI) regulations and has adopted GS1 Standards to ensure compliance with the regulations.
Luminex is adhering to the compliance timelines and expectations as specified by the FDA’s final rule on UDI.
Actions and Timelines
In mid-late August 2016, Luminex will begin labeling products for all Class I and Class II IVD devices under the new UDI regulations. All future IVD products developed by Luminex that fall within scope of the FDA UDI final rule will have also have updated labels to meet FDA regulations.
| Compliance Date | Requirement |
|---|---|
| 1 year after publication of the final rule (September 24, 2014) |
Class III medical devices. Data must be submitted to GUDID. New date format must be implemented. |
| 2 years after publication of the final rule (September 24, 2015) |
Class II implantable, life-supporting, and life-sustaining devices. Data must be submitted to GUDID. New date format must be implemented. |
| 3 years after publication of the final rule (September 24, 2016) |
Class II medical devices must bear a UDI. Data must be submitted to GUDID. New date format must be implemented. |
| 5 years after publication of the final rule (September 24, 2018) |
Class I medical devices and devices that have not been classified into class I, class II, or class III must bear a UDI.
Dates on the labels of all devices, including devices that have been exempted from UDI labeling requirements. |
| 7 years after publication of the final rule (September 24, 2020) |
Class I devices, and devices that have not been classified into class I, class II, or class III that are required to be labeled with a UDI, must bear UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. |
| Ref- www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/UniqueDeviceIdentification/CompliancedatesforUDIRequirements/ | |
Label Format
What is changing? See below for a comparison of xTAG®, NxTAG®, Multicode®, ARIES®, xMAP® labeling changes.
xTAG® Old Label Format Example

xTAG® New Label Format Example

NxTAG® Old Label Format Example
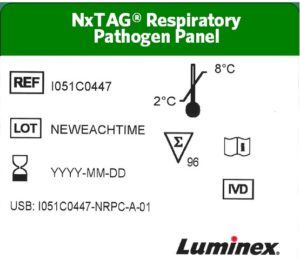
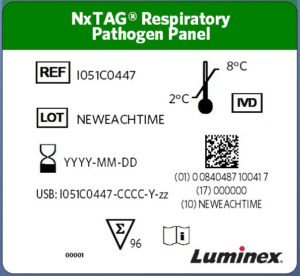
NxTAG® New Label Format Example
xMAP® Old Label Format Example

xMAP® New Label Format Example

MultiCode® Old Label Format Example

MultiCode® New Label Format Example

ARIES® Old Label Format Example

ARIES® New Label Format Example

Summary of Changes
Date format changed: From YYYY-MM , To: YYYY-MM-DD (where applicable)
Addition of GS1 DataMatrix Barcode Information:
(01) – GTIN # (UDI DI #)
(11) – Manufacturing/Production Date (YYMMDD) (where applicable)
(17) – Expiration Date (YYMMDD) (where applicable)
(10) – Lot Number (where applicable)
(21) – Serial Number (where applicable)
Helpful Links
FDA Final Rule
FDA – Unique Device Identification – UDI
FDA – Global UDI Database (GUDID)
GS1 US and FDA UDI