
Improves Outcomes
The VERIGENE® Bloodstream Infection portfolio empowers laboratories to rapidly identify causative pathogens, as well as their associated resistance markers.
Clinical studies have demonstrated that the VERIGENE Bloodstream Infection assays lower overall hospital costs, shorten the patient’s length of stay, and most importantly, improve patient outcomes by enabling a more targeted treatment plan—faster.1 These assays have been proven to synergize with antimicrobial stewardship programs, reduce the use of unnecessary antibiotics, and effectively manage infection control.1,2

Panel Menu
The VERIGENE Bloodstream Infection portfolio provides accurate test results in a clinically meaningful timeframe.
Gram-Positive Blood Culture Test (BC-GP)
Species
Staphylococcus aureus
Staphylococcus epidermidis
Staphylococcus lugdunensis
Streptococcus agalactiae
Streptococcus pneumoniae
Streptococcus pyogenes
Enterococcus faecalis
Enterococcus faecium
Group
Streptococcus anginosus
Genus
Staphylococcus spp.
Streptococcus spp.
Micrococcus spp.+
Listeria spp.
Resistance
mecA (methicillin)
vanA (vancomycin)
vanB (vancomycin)

+Micrococcus spp. is not U.S./FDA-cleared
Gram-Negative Blood Culture Test (BC-GN)
Species
Escherichia coli*
Klebsiella pneumoniae
Klebsiella oxytoca
Pseudomonas aeruginosa
Serratia marcescens++
Genus
Acinetobacter spp.
Citrobacter spp.
Enterobacter spp.
Proteus spp.
Resistance
CTX-M (ESBL)
IMP (carbapenemase)
KPC (carbapenemase)
NDM (carbapenemase)
OXA (carbapenemase)
VIM (carbapenemase)

* BC-GN will not distinguish Escherichia coli from Shigella spp. (S. dysenteriae, S. flexneri, S. boydii, and S. sonnei).
++Serratia marcescens is not U.S./FDA-cleared

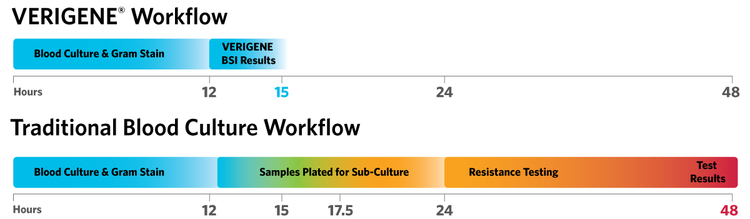
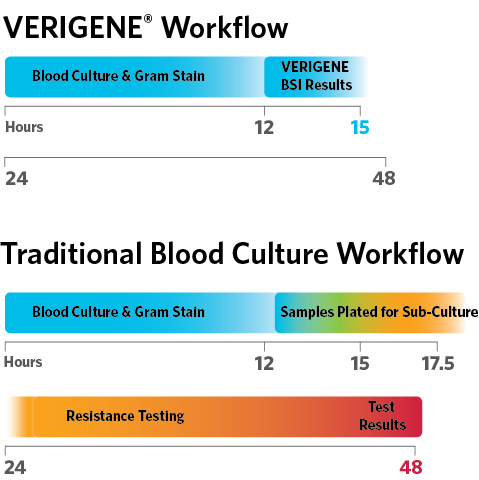
Sample to Answer
Time to results is 3x faster using the VERIGENE® System versus traditional methods.

Reduces Costs
The VERIGENE Bloodstream Infection assays enable healthcare professionals to optimize therapy earlier, which has been shown to reduce the length of inpatient care, lowering overall healthcare costs.1,2
- Rivard K, Athans V, Lam S, et al. Impact of antimicrobial stewardship and rapid microarray testing on patients with Gram-negative bacteremia. Eur J Clin Microbiol Infect Dis. 2017 Oct;36(10):1879-87. doi: 10.1007/s10096-017-3008-6.
- Box M, Sullivan E, Ortwine K, et al. Outcomes of rapid identification for Gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy. 2015; 35(3): 269-276.
海外参考情報
Product Information:
- VERIGENE® Gram-Positive Blood Culture Nucleic Acid Test (BC-GP) (IVD)
- VERIGENE® Gram-Negative Blood Culture Nucleic Acid Test (BC-GN) (IVD)
Peer-Reviewed Publications:
- Impact of a Rapid Blood Culture Assay for Gram-Positive Identification and Detection of Resistance Markers in a Pediatric Hospital – Archives of Pathology & Laboratory Medicine
- Impact of Antimicrobial Stewardship and Rapid Microarray Testing on Patients with Gram-Negative Bacteremia – European Journal of Clinical Microbiology & Infectious Diseases
- Identification of Gram-Negative Bacteria and Genetic Resistance Determinants from Positive Blood Culture Broths by Use of the VERIGENE Gram-Negative Blood Culture Multiplex Microarray-Based Molecular Assay – Journal of Clinical Microbiology
- Clinical Impact of Laboratory Implementation of Verigene BC-GN Microarray-Based Assay for Detection of Gram-Negative Bacteria in Positive Blood Cultures – Journal of Clinical Microbiology
Posters:
- Clinical Impact After Laboratory Implementation of the Verigene Gram-Negative Bacteria Microarray for Positive Blood Cultures
- Use of the VERIGENE Gram Negative Blood Culture (BC-GN) Test for More Rapid Bacterial Identification and Antimicrobial Optimization
Blogs:
- Investment Needed in Antimicrobial Stewardship
- Leading Pediatrics Hospital Finds Rapid Testing Reduces Unnecessary Use of Antibiotics
- The Pathologist: Labs Embrace Rapid Testing to Address Antibiotic Resistance
- How a Community Hospital Saved $600,000 with Rapid Diagnostics
Looking for additional documentation and publications?
Documentation Search Publications Search海外参考情報
Webinar: Strategic Implementation of a Rapid Molecular Blood Culture Panel for Gram Negative Bacteremia
Webinar: Rapid Diagnostic Testing of Positive Blood Cultures & Impact on Antimicrobial Stewardship
Webinar: The Clinical and Economic Case for Rapid Sepsis Diagnostics
Webinar: Optimizing Therapy for Bloodstream Infections with Rapid Molecular Testing
Webinar: Improving Management of CRE’s and Other Antibiotic-Resistant BSI’s with Rapid MDx
Webinar: Physician and Pharmacist Perspectives on the Value of Rapid Blood Culture Testing
Webinar: Best Practices for Multiplex Molecular Infectious Disease
Parkview Medical Center & VERIGENE® Testimonial
Med Fusion & VERIGENE®
Testimonial
Mission Pathogen: Luminex Virtual Reality Experience – Bloodstream Infection
For In Vitro Diagnostic Use. Products are region specific and may not be approved in some countries/regions. Please contact Luminex at support@luminexcorp.com to obtain the appropriate product information for your country of residence.
Intended Use
The VERIGENE® Gram-Positive Blood Culture Nucleic Acid Test (BC-GP) performed using the sample-to-result VERIGENE System is a qualitative, multiplexed in vitro diagnostic test for the simultaneous detection and identification of potentially pathogenic gram-positive bacteria which may cause bloodstream infection (BSI). BC-GP is performed directly on blood culture bottles identified as positive by a continuous monitoring blood culture system and which contain gram-positive bacteria.
BC-GP detects and identifies the following bacterial genera and species: Staphylococcus spp., Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus lugdunensis, Streptococcus spp., Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus anginosus group, Enterococcus faecalis, Enterococcus faecium, and Listeria spp.
In addition, BC-GP detects the mecA resistance marker, inferring mecA-mediated methicillin resistance, and the vanA and vanB resistance markers, inferring vanA/vanB-mediated vancomycin resistance. In mixed growth, BC-GP does not specifically attribute van-mediated vancomycin resistance to either E. faecalis or E. faecium, or mecA-mediated methicillin resistance to either S. aureusor or S. epidermidis.
BC-GP is indicated for use in conjunction with other clinical and laboratory findings to aid in the diagnosis of bacterial bloodstream infections; however, is not to be used to monitor these infections. Sub-culturing of positive blood cultures is necessary to recover organisms for susceptibility testing, identification of organisms not detected by BC-GP, differentiation of mixed growth, association of antimicrobial resistance marker genes to a specific organism, or for epidemiological typing.
+Micrococcus spp. is not U.S./FDA-cleared
The VERIGENE® Gram-Negative Blood Culture Nucleic Acid Test (BC-GN) performed using the sample-to-result VERIGENE® System is a qualitative multiplexed in vitro diagnostic test for the simultaneous detection and identification of selected gram-negative bacteria and resistance markers. BC-GN is performed directly on blood culture media using blood culture bottles identified as positive by a continuous monitoring blood culture system and which contain gram-negative bacteria as determined by Gram stain.
BC-GN detects and identifies the following Bacterial Genera and Species: Acinetobacter spp., Citrobacter spp., Enterobacter spp., Proteus spp., Escherichia coli1, Klebsiella pneumoniae, Klebsiella oxytoca, and Pseudomonas aeruginosa, and Resistance Markers: CTX-M (blaCTX-M), KPC (blaKPC), NDM (blaNDM), VIM (blaVIM), IMP (blaIMP), and OXA (blaOXA).
BC-GN is indicated for use in conjunction with other clinical and laboratory findings to aid in the diagnosis of bacterial bloodstream infections; however, is not used to monitor these infections. Sub-culturing of positive blood cultures is necessary to recover organisms for antimicrobial susceptibility testing (AST), for identification of organisms not detected by BC-GN, to detect mixed infections that may not be detected by BC-GN, for association of antimicrobial resistance marker genes to a specific organism, or for epidemiological typing.
* BC-GN will not distinguish Escherichia coli from Shigella spp. (S. dysenteriae, S. flexneri, S. boydii, and S. sonnei).
++Serratia marcescens is not U.S./FDA-cleared









