Have you ever wondered about the technology that helps runs your tests? We’ve provided you with a little more information about our bead products, and how they can help you!
Luminex beads (aka microspheres) make our technology one-of-a-kind. Microplex® microspheres are 5.6 µm in diameter and are made of polysterene. The small size allows for easy suspension in liquid, resulting in fast kinetics behavior.
Our MagPlex® beads are derived from embedding the standard Microplex® microspheres with superparamagnetic particles. They retain the same chemistry as the Microplex® beads, with the carboxyl groups on the surface and two internal dyes; however, the MagPlex® beads are larger at 6.5 microns in diameter. The magnetic properties allow the MagPlex® beads to move quickly toward the magnetic field and re-suspend easily upon removal of the field; thereby, eliminating filtration steps in the assays.
Microspheres are dyed by placing them in an organic solution containing two fluorescent dyes, one emitting red wavelengths and one emitting infrared wavelengths. In the organic solution, the microspheres swell, allowing the dyes to diffuse into the microsphere. The microspheres are then washed, thereby shrinking the microspheres to their original size allowing the dyes to get trapped in the microsphere interior. Each bead is distinguished by its unique concentration of red to infrared dye.
Microspheres are light-sensitive. Four to six hours of cumulative exposure to light will cause microspheres to fall out of their proper regions due to photobleaching of the internal dyes. The damage is irreversible and microspheres must be discarded. Protect microspheres from light during incubations and while in storage. If many microspheres appear in the upper right of the region, it is a sign of agglutination (or sticking) of microspheres. This is usually due to highly hypdrophobic molecules sticking to the microspheres and can be minimized through the use of vortexing/sonicating, detergents and blocking agents.
A normal bead detail display is shown below. It depicts a tight bead population within a white region:
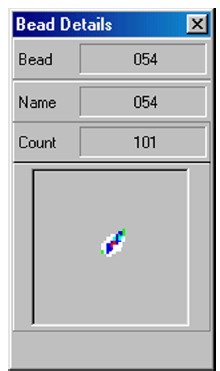
It is good practice to review calibration/control trend reports periodically to detect trends.
Use xMAP® control microspheres to check the success of the system calibration and for troubleshooting purposes. If there is a problem with your kit results, xMap controls can help determine if the problem is a hardware issue.
Below are some tips to help in calibration:
| Symptom | Possible Problem | Solution |
|---|---|---|
xMAP microspheres classify too high. |
You may be using photo bleached calibration microspheres. | Replace the calibration microspheres with a fresh batch. To avoid photobleaching, protect your microspheres from light. |
xMAP microspheres hit the lower right of the region. |
You may be using photo bleached xMAP microspheres. | Replace the microspheres with a fresh batch. To avoid photobleaching, protect your microspheres from light. |
Beads appear scattered. |
There is air in the system. | Verify sample probe height. Turn three Prime commands, two Alcohol Flush commands, and then three Wash commands with distilled water. |
Beads appear scattered.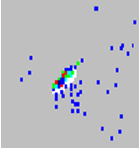 |
The sheath fluid is empty. | Make sure there is sheath fluid in the sheath container. Prime the system until all air is out of the system. |
Microspheres appear as a long diagonal line.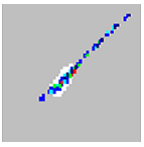 |
The xMAP microspheres have agglutinated. | Add additional detergent to the assay buffer. For example, add 02% to 0.1% Tween-20, Triton X100, or SDS. |
Microspheres appear as a long diagonal line. |
The solvent is incompatible. | Contact Luminex Technical Support for a list of incompatible solvents. If the solvent you are using is listed, switch solvents. |
 |
You are using incompatible sheath fluid. | Use only Luminex sheath fluid in the Luminex® 200™ analyzer. Other fluids may damage your analyzer and may void your warranty. |