The pre-treatment step of your stool sample is key for optimal performance of the GPP assay. The “pre-treatment” refers to the crucial step just prior to extraction whereby we break up the stool and mechanically lyse any parasites, bacteria, or viruses present in the sample. This pre-treatment step is critical in ensuring maximum extraction efficiency, especially for parasitic targets which may require more than just a chemical lysis.
The amount of stool that is added to the pre-treatment step is important. Adding 100–150 mg of solid stool or 100 μL of liquid stool is the most variable aspect of the assay due to the variability of the sample.
Since it’s unlikely that you will weigh each solid sample, using a standard 10 μL microbiology loop to pick up the sample (about a couscous grain size) will help in measuring a consistent volume (see the picture below). Although your instinct will be to add more sample, remember that less is more. Adding too much of the sample will result in lower signals due to the increased level of inhibitors present.
For liquid samples, you may need to cut the tip of a P1000 pipette to pick up 100 μL of sample. We recommend using extra-long tips (e.g. Molecular Bio Products REACH tips) to prevent contamination of your pipette.
The diagram below illustrates the pre-treatment procedure for fresh or frozen stool sample:
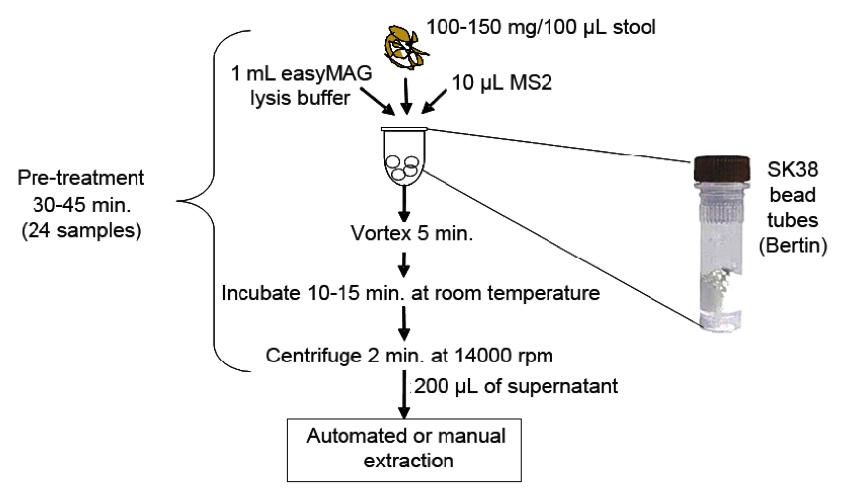
Add 1 mL of the lysis buffer to the Bertin Tube (Bertin® #03961SK38), then add 10 mL of the MS2.
Adding the MS2 directly to the lysis buffer stabilizes the MS2 prior to addition of the stool in case there are any nucleases present in the stool that could degrade the MS2. Now add the appropriate amount of stool as described above.
The tube containing the beads and the reagents is now ready for a good beating. This step is necessary in order to break up the stool. We recommend vortexing for a full five minutes. Using a MoBio 24 tube vortex adapter (Mo-Bio® # 13000-V1-24) will free up your hands, so you don’t need to hold the tubes during the beating process. We don’t have recommendations for other vortex tube holders, (i.e., a vertical model versus the horizontal MoBio), but as long as the tubes are held securely and a good vortex is obtained, it should work.
After the 5 minutes of beating, incubate the tubes at room temperature for 10–15 minutes.
Next, centrifuge the tubes at 14,000 RPM for 2 minutes. This is equal to 20,800 RCF. This step is performed to pellet the larger debris. All cells should be lysed at this point due to the bead beating in combination with the lysis buffer. The nucleic acid should stay in the supernatant. The supernatant is now put through the normal extraction process to completely purify the nucleic acids and remove the inhibitors that may prevent amplification.
When removing the supernatant from the Bertin tubes, there may be a lipid layer on the top. You want to avoid this layer by piercing through it and aspirating only the supernatant above the beads. You do not want to aspirate any of the beads or other pelleted debris because they can clog the extractor tips (if using system like an easyMAG).
The pre-treated sample can be stored in the Bertin tubes after extraction in a -80⁰C freezer. At best, you can get four extractions from 1 pre-treatment; however, the average is normally three extractions.
As you can see, the pre-treatment step is a simple yet critical process in obtaining optimal results from your stool samples.
NOTE: Cultured material can be extracted with or without sample pre-treatment. Use 200 μL of culture material directly and follow manufacturer’s specifications for a given extraction method.