You’ve been using xTAG assays in your lab for a while, and now you’re ready to bring on xTAG® Gastrointestinal Pathogen Panel (GPP) to detect viruses, bacteria, and parasites that cause gastroenteritis. If you want to properly differentiate and identify these pathogens, it is critical that you also differentiate GPP from other assays.
You already know the importance of pre-treating stool samples for GPP, and you’ve surely mastered the fine art of poop-scooping. You also know that you should never interchange reagents between different assay kits (for example, the reporter buffer and SAPE for GPP and RVP are different formulations). Today we’ll explore another major difference: GPP’s use of FAST chemistry and how it simplifies workflow and reduces turnaround time.
How Does it Work, and How Much Faster?
As a seasoned xTAG practitioner, you are aware of the traditional procedure: Extraction > Multiplex PCR > Amplicon Treatment > Allele-/Target-Specific Primer Extension > Bead Hybridization > Reporter Addition > Detection. Similarly, GPP utilizes a condensed version of this chemistry. Here’s an overview of the FAST workflow:

The first thing you’ll notice is the streamlined process, hence the FAST moniker. GPP can be performed in as little as five hours. This turnaround time is favorable when compared to other diagnostic methods such as stool culture or ova and parasite (O&P) exams.
As you dig deeper into the actual procedure, you may ask yourself, “Where’s the amplicon treatment and target-specific primer extension (TSPE)?” Before we get to the answer, let’s examine the purpose of each step.
Amplicon treatment is used to remove any unincorporated primers and dNTPs from the RT-PCR products. Removing these items is essential because the TSPE step will introduce new primers—with a TAG sequence incorporated at the 5’ end—and new dNTPS whose dCTPs are biotinylated. These TAGs and biotinylated-cytosines will allow us to later attach our beads and reporter molecules, respectively. Together, amplicon treatment and TSPE consume about two hours of thermal cycling time. To eliminate these separate reactions but maintain TSPE’s functionality, we have developed target-specific RT-PCR (illustrated below).
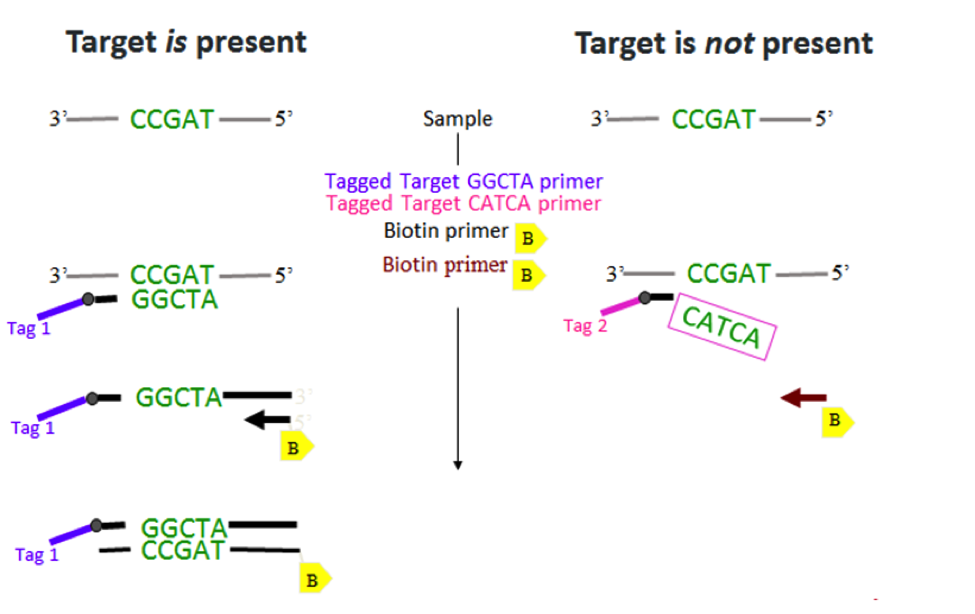
With this chemistry, the TAG sequences and biotin are now introduced during the initial RT-PCR step. Just like TSPE, a primer has a tag sequence on its 5’ end. But instead of using dCTPs, biotin is now incorporated on the 5’ end of the reverse primer. If the target is present, the double-stranded RT-PCR product will include a TAG on one end and biotin on the other. If the target is not present, the TAG and biotin will not be incorporated because extension will not occur.
These RT-PCR products are already equipped for bead hybridization. At this step there is another added bonus: the bead hybridization can be consolidated with reporter addition into one simple step. By the time this incubation is complete, the TAGs are coupled to their respective beads and the biotin has bonded with the reporter conjugate. You are now ready for data acquisition on a pre-heated analyzer. GPP is as simple and FAST as that!
Not So FAST, My Friend
Before you start thanking us for this enhanced chemistry (or questioning why we didn’t develop it sooner—or, dare I say, faster) we must discuss some precautions that must be taken when performing GPP. By consolidating many reactions with FAST chemistry, there are many molecular interactions occurring simultaneously. This can create opportunity for non-specific binding and the potential for false positive results. In contrast, it must also be noted that FAST chemistry reduces your chances of contamination by eliminating multiple manual handling steps. Nevertheless, these GPP precautions are universal for molecular assays but are of heightened significance with FAST chemistry.
1. Maintain unidirectional workflow
Designate rooms and/or workbenches for pre-PCR, template addition and amplification. Each area should have dedicated supplies and equipment.
2. Pay close attention to temperature
Reagents and samples should always be handled and/or stored at the appropriate temperatures and conditions specified by the package insert.
- Keep PCR reagents on a cold block when preparing master mix, especially the xTAG OneStep Enzyme.
- Keep prepared master mix and extracted samples cold during template addition.
- Pre-heat the thermal cycler to the specified temperatures for RT-PCR and bead hybridization steps.
Pre-heat the LX200 analyzer before data acquisition.
3. Work in an efficient, timely manner
Organize workbenches, supplies and techniques to minimize the time between hands-on steps.
- After extraction, transfer nucleic acid eluates to strip tubes or PCR plates instead of tubes that require individual manipulation.
- Use a repeater pipette to aliquot master mix and beads, especially for larger runs.
- Use multichannel pipettes for:
- Transferring extracted samples to master mix.
- Adding PCR products to beads.
- Adding and mixing reporter solution.
Let’s Quickly Wrap This Up
By employing xTAG GPP to detect gastroenteritis-causing pathogens, you can simplify labor for your lab and reduce turnaround time for your patients. Like all technological advancements, its FAST chemistry also poses its own set of challenges. However, if you follow the tips outlined above and do not deviate from the package insert, you can easily mitigate your chances for erroneous results. You should now be prepared to take on those potentially-infectious stool samples without trepidation.